Béla Gyurcsik1
1 Department of Molecular and Analytical Chemistry, University of Szeged, Dóm tér 7-8, H-6720 Szeged, Hungary
gyurcsik@chem.u-szeged.hu
streda 12. júna 2024 14:00
knižnica Oddelenia anorganickej chémie, č.d. 358
Fakulta chemickej a potravinárskej technológie STU BA
Radlinského 9
81237 Bratislava
Online prednáška cez MS Teams
Záznam prednášky si môžete pozrieť na YouTube kanáli Chemické horizonty https://youtu.be/LURrtR9F9PQ
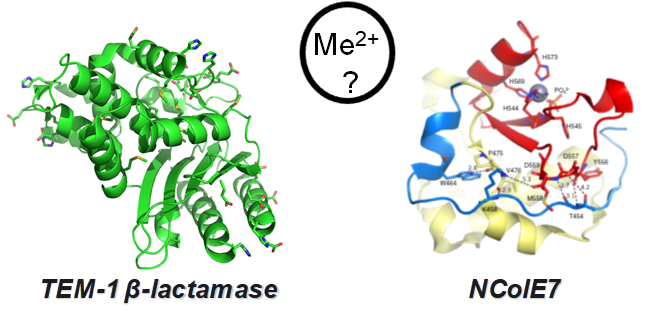
The lecture includes two types of bacterial defence systems. (1) TEM-1 β-lactamase, protecting bacteria from β-lactam antibiotics. While this is not a metalloenzyme, it offers potential sites for metal ion binding. Recently, we have studied the effect of Ni(II), Cd(II), and Hg(II) on the structure and catalytic activity of TEM-1 β-lactamase. Interaction of TEM-1 β-lactamase surface histidines with Ni(II) was proven by immobilized Ni(II) ion affinity chromatography. While the sulphur-containing methionine residues may coordinate soft metal ions, only a slight change was observed in the circular dichroism spectra of the enzyme in their presence. Mass spectrometry and 119mHg perturbed angular correlation spectroscopy revealed weak binding of Hg(II). To describe the catalysed hydrolytic process of ampicillin, slow conversion of the primary reaction product needed to be considered. Ni(II) and Cd(II) promoted the catalytic activity of the enzyme, while Hg(II) exerted inhibitory effect. Hg(II) and Ni(II) inhibited the growth of E. coli, but addition of ampicillin could neutralize this toxic effect by complexing the metal ions. (2) NColE7 is the nuclease domain of the colicin E7 bacterial toxin. It is a metalloenzyme, which purifies together with Zn(II) ions. Surprisingly, the apo form is more active than the Zn(II)-bound form. Addition 1 eq of Cu(II) ions to the apo-enzyme has similar effect, while 1 eq of Cd(II) ions did not decrease the catalytic to the same extent due to the lower affinity towards the catalytic site than Cu(II). In contrast, the enzyme has extremely high activity in the presence of 1 eq of Ni(II) ion, while the excess of the Ni(II) ion could not significantly inhibit this highly active enzyme. Our findings suggest that the non-native metal ions may modify the mechanism of the substrate hydrolysis – providing a chance to design more efficient antibiotic compounds.
